
 Location:Home/News/Company News
Location:Home/News/Company NewsOriginal article by Lao Yang666 | Talks on Pharmaceutical Chemistry January 01, 2025, 21:23, Shanghai
The year 2024 can be described as a challenging 'winter' for the pharmaceutical industry, with no end in sight. Nevertheless, several self-developed small-molecule drugs were successfully launched in China in 2024. Below is a brief overview.
I. Ganagliflozin Proline Tablets
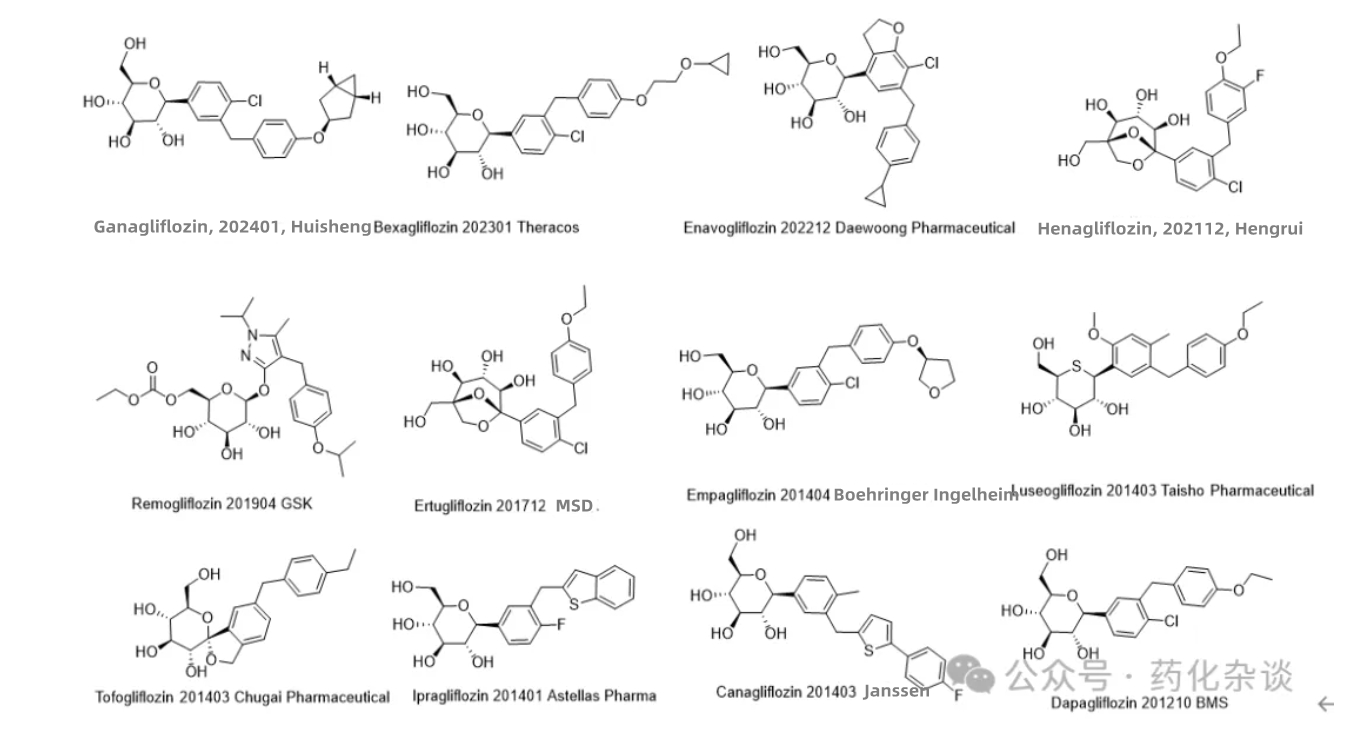
Ganagliflozin Proline Tablets, an SGLT-2 inhibitor developed by Huisheng Biopharmaceutical, was approved in January 2024. This compound works by reducing renal tubular reabsorption of glucose, thereby lowering blood sugar levels. To date, 12 drugs targeting this site have been approved for market entry. The structures, launch timelines, and respective R&D companies are detailed in the table below. From a structural perspective, Ganagliflozin shares significant similarity with Dapagliflozin (BMS), Empagliflozin (Boehringer Ingelheim), and Bexagliflozin (Theracos), with the closest resemblance to Empagliflozin. Henagliflozin
II. Tegileridine Fumarate
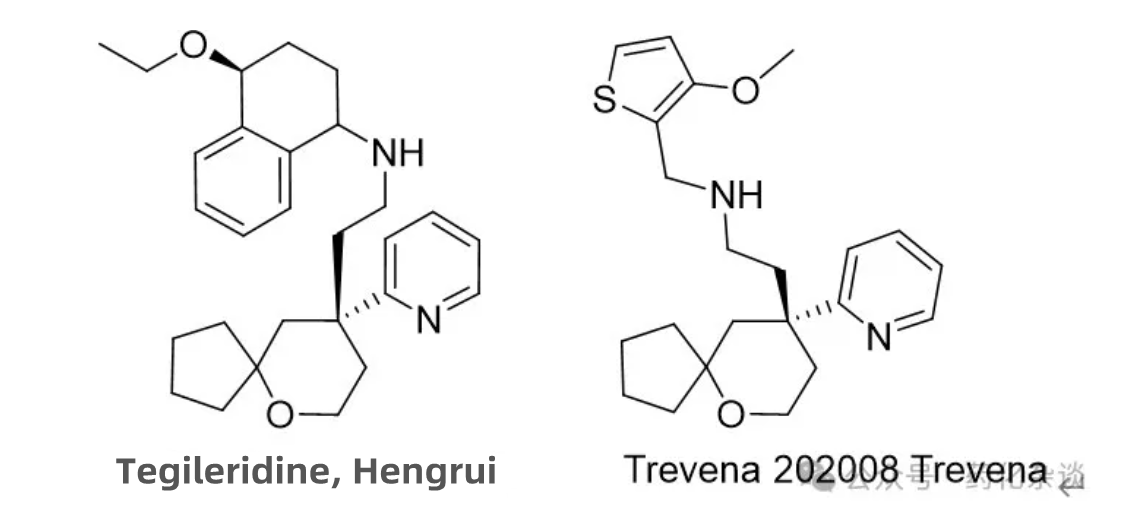
Tegileridine Fumarate, developed by Hengrui Pharmaceuticals and approved in January 2024, is a μ-opioid receptor (MOR) agonist indicated for the treatment of moderate to severe pain after abdominal surgery. There are various structures of drugs acting on the MOR, among which only Trevena, approved in August 2020, shares a similar structure to Tegileridine.
III. Tunlametinib
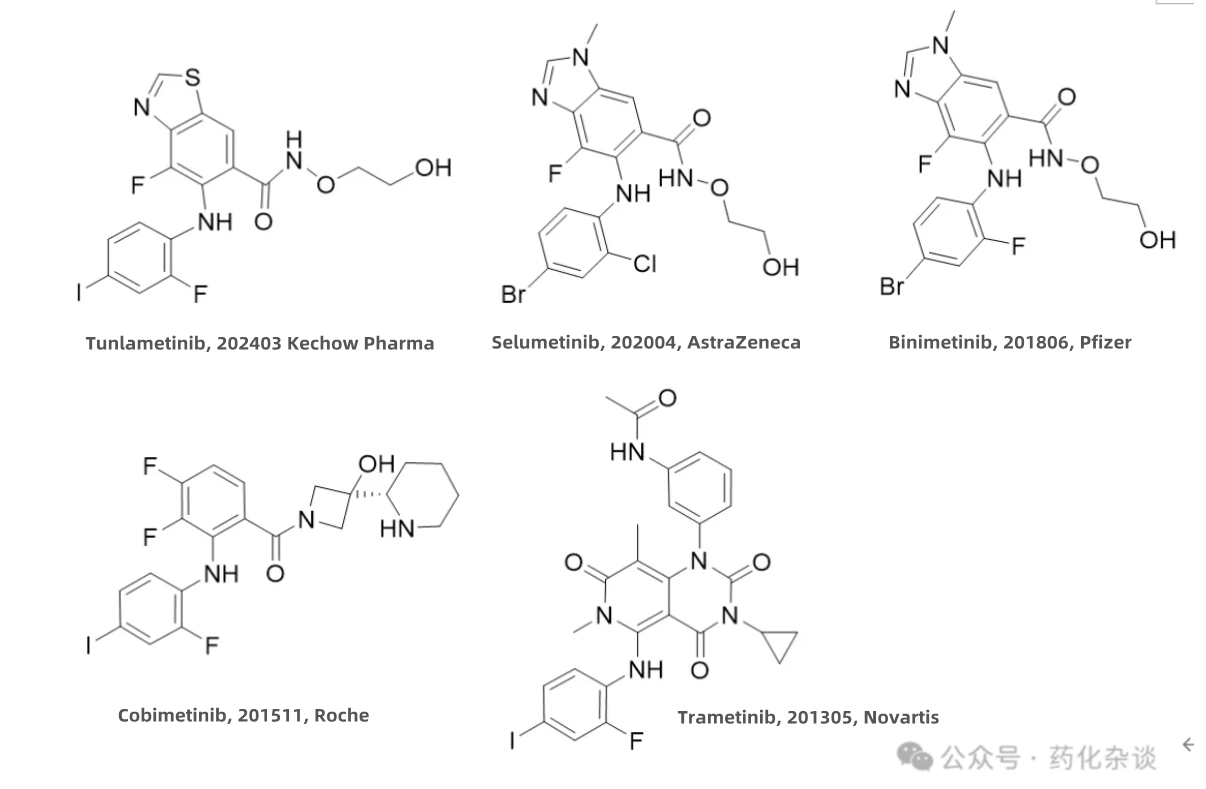
Tunlametinib, a MEK inhibitor developed by Kechow Pharma, was approved in March 2024 for the treatment of advanced NRAS-mutant melanoma in patients who have failed PD-1/PD-L1 therapy. Structurally, Tunlametinib is similar to Selumetinib (AstraZeneca) and Binimetinib (Pfizer), with the primary difference being that the benzimidazole ring in its structure is replaced by a benzothiazole ring. Selumetinib, in contrast, only differs from Binimetinib in substituting a fluorine atom with a chlorine atom.
IV. Unecritinib
V. Envonalkib
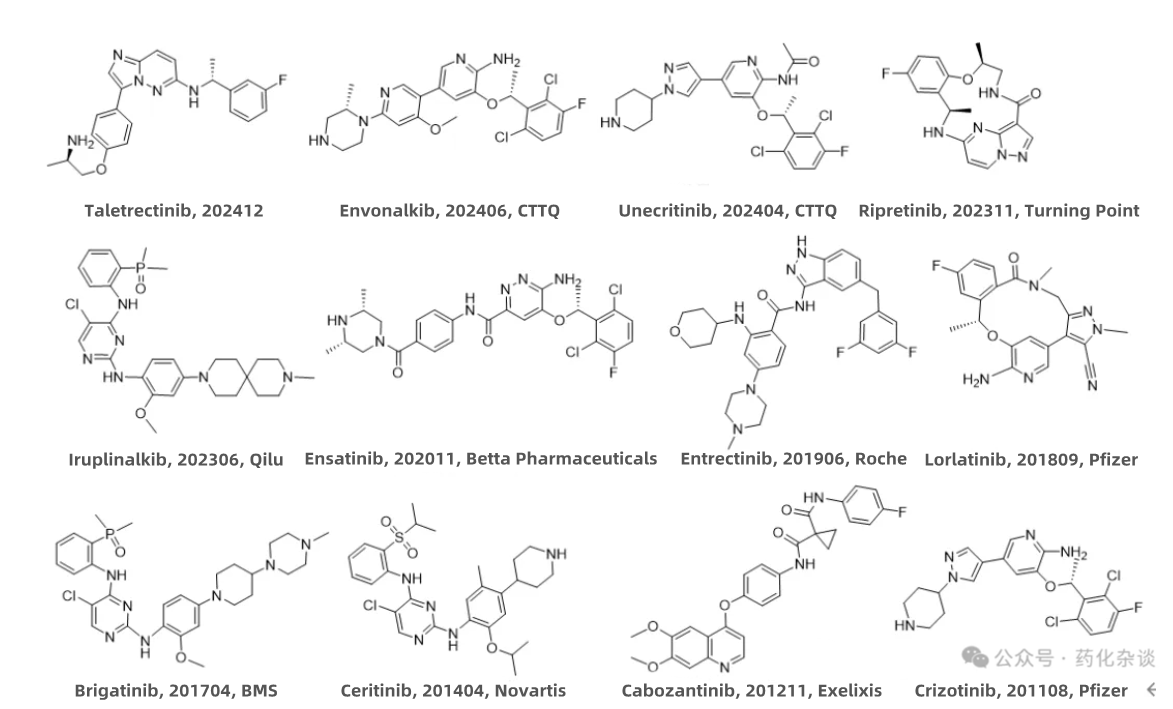
Unecritinib, a ROS1 inhibitor developed by CTTQ, was approved in April 2024 for the treatment of adult patients with ROS1+ locally advanced or metastatic non-small cell lung cancer (NSCLC). Envonalkib, an ALK inhibitor developed by CTTQ, was approved in June 2024 for the treatment of ALK+ locally advanced or metastatic non-small cell lung cancer (NSCLC) in patients naïve to ALK inhibitor therapy. Drugs targeting these two pathways share similar indications and are often multi-targeting agents, typically capable of simultaneously inhibiting ROS1 and ALK, among other targets. Structurally, these two drugs share a similar structure, resembling Ensatinib (Betta Pharmaceuticals) and Crizotinib (Pfizer).
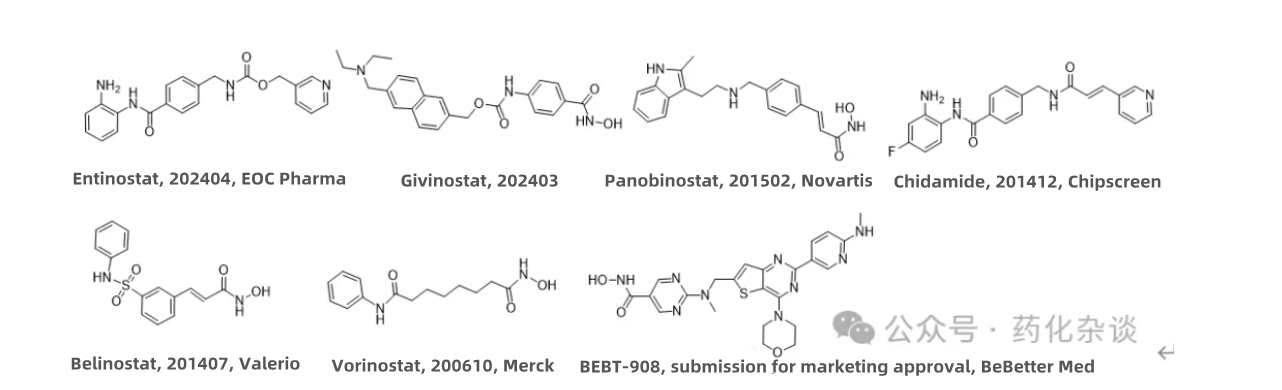
VI. Entinostat
Entinostat, an HDAC inhibitor developed by EOC Pharma, was approved in April 2024 for use in combination with aromatase inhibitors in the treatment of HR+/HER2- locally advanced or metastatic breast cancer that has recurred or progressed after endocrine therapy. Compared to other HDAC inhibitors, this drug selectively inhibits class I HDAC enzymes and features a longer half-life. Its structure is quite similar to Chidamide (Chipscreen).
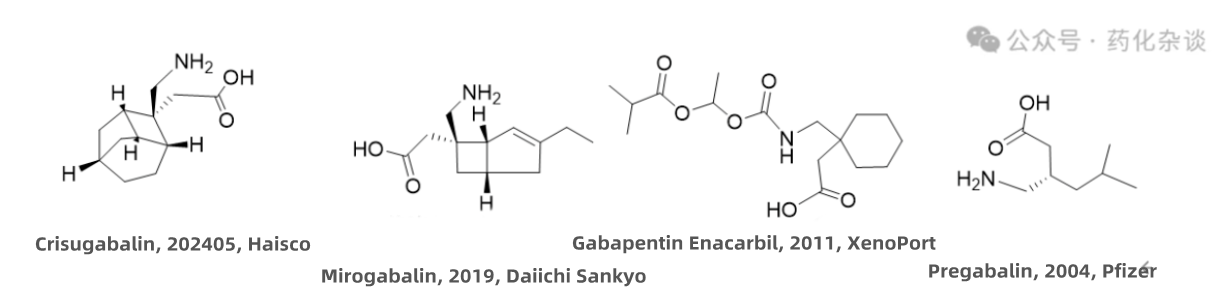
VII. Crisugabalin Besilate
Crisugabalin Besilate, a third-generation central nervous system calcium channel modulator developed by Haisco, was approved in May 2024. As a γ-aminobutyric acid (GABA) analogue, it is indicated for the treatment of diabetic peripheral neuropathic pain in adults. This drug is characterized by a relatively unique tricyclic structure.
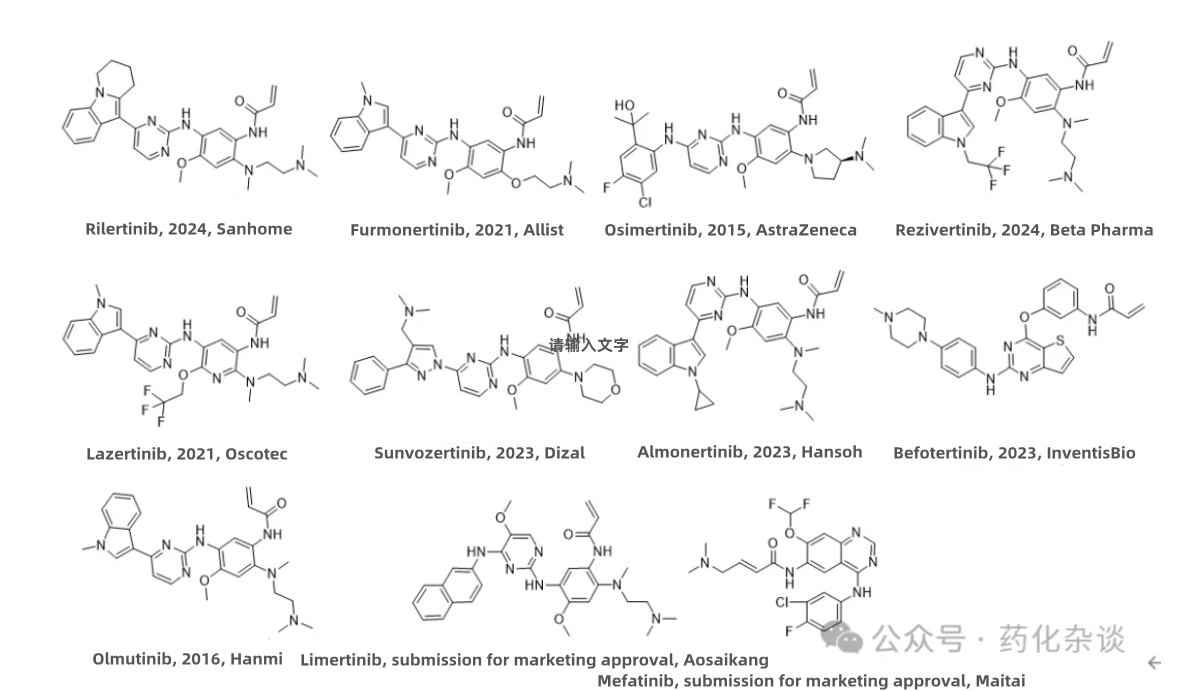
VIII. Rezivertinib
IX. Rilertinib
Rezivertinib, developed by Beta Pharma and approved in May 2024, and Rilertinib, developed by Sanhome and approved in 2024, are both third-generation EGFR inhibitors indicated for the treatment of locally advanced or metastatic non-small cell lung cancer (NSCLC) in adults who are positive for the EGFR T790M mutation. The structures of these two drugs are strikingly similar, and they belong to a group of structurally related compounds that trace back to Osimertinib, which was launched in 2015.
X. Golidocitinib
Golidocitinib, a highly selective JAK1 inhibitor developed by Dizal Pharma, was approved in June 2024 for the treatment of adult patients with relapsed or refractory peripheral T-cell lymphoma (r/r PTCL) who have received at least first-line systemic therapy. Although several JAK1 inhibitors are available on the market, their selectivity often remains suboptimal. Golidocitinib stands out as one focusing on tumor therapy, while current research mainly emphasizes anti-inflammatory applications.

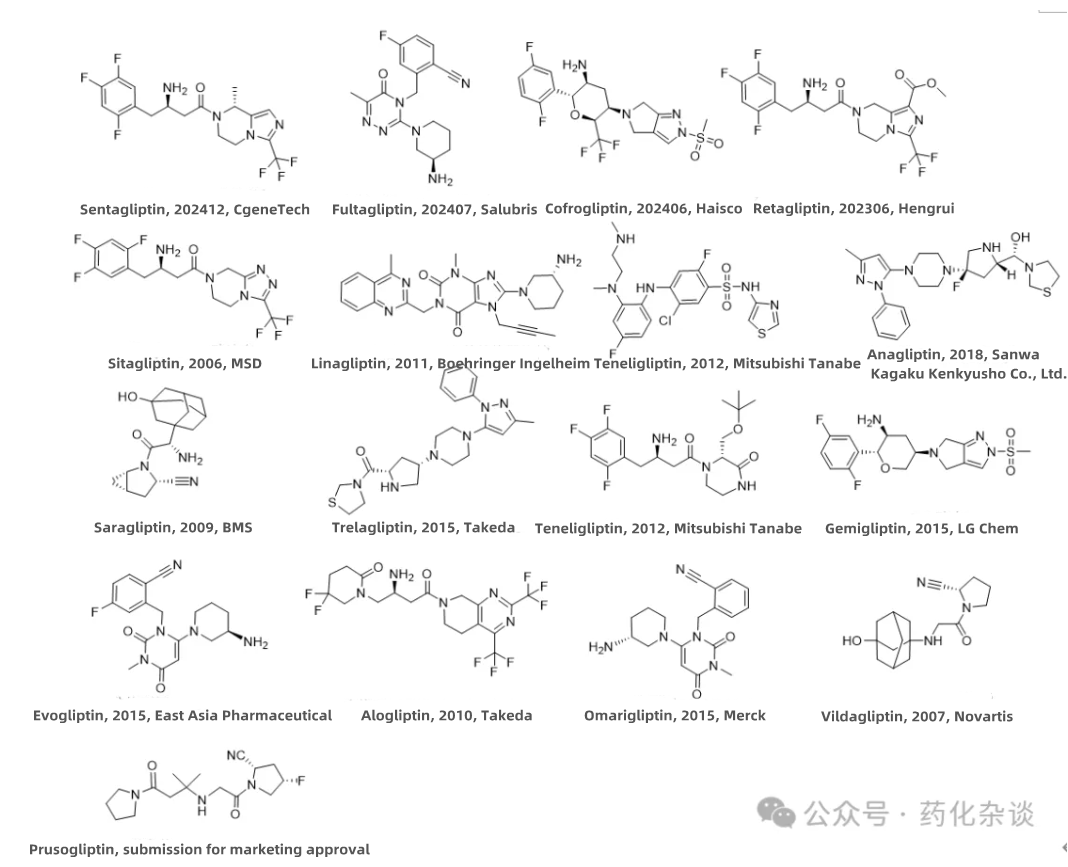
XI. Cofrogliptin
XII. Sentagliptin
XIII. Fultagliptin
Cofrogliptin (developed by Haisco and approved in June 2024), Sentagliptin (developed by CGeneTech and approved in December 2024), and Fultagliptin (developed by Salubris and approved in July 2024) are all DPP-4 inhibitors used to improve glycemic control in adult patients with type 2 diabetes mellitus (T2DM). Cofrogliptin is derived by introducing a trifluoromethyl group into Omarigliptin. Sentagliptin is produced by introducing a methyl group into Sitagliptin, and Retagliptin (Hengrui Pharmaceuticals) shares a similar structure. Fultagliptin has a structural similarity to both Alogliptin and Trelagliptin.
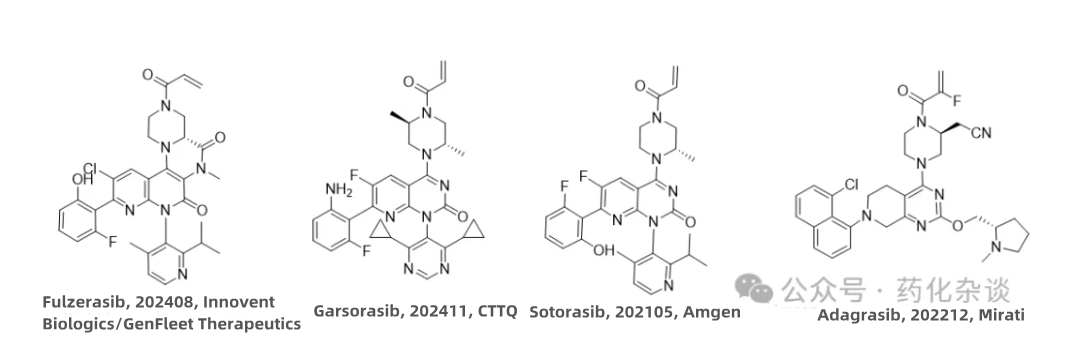
XIV. Fulzerasib
XV. Garsorasib
Fulzerasib (a KRAS G12C inhibitor developed by Innovent Biologics and GenFleet Therapeutics and approved in August 2024) and Garsorasib (a KRAS G12C inhibitor developed by CTTQ and approved in November 2024) are both intended for the treatment of advanced non-small cell lung cancer (NSCLC) in adult patients with KRAS G12C mutations. From a structural perspective, both drugs are derivatives of Sotorasib.
The harsh winter will inevitably pass as the industry continues to grow. We hope that Chinese companies will evolve from being followers to becoming pioneers in the future.